Lab Informatics Resources
Free tools and resources on science-aware™ lab informatics.
Optimizing Clinical Diagnostic Lab Workflows For LabCorp
This article is based on a webinar presented by LabCorp’s Angela Kenyon. Click here to view to entire webinar.
For over 50 years, LabCorp has produced discoveries and insights that help make real, meaningful differences for people around the world. Its world-class diagnostic tests give healthcare providers insights that help enable and accelerate a patient’s care and access to innovative treatments, medicines, and new technologies that can change outcomes and lives.
Dry and Wet Testing Workflows in the Clinical Lab
LabCorp is substantially and meaningfully involved in the diagnostic process that typically starts with a patient’s medical consultation, proceeds through specimen collection and testing, and culminates with test results. At LabCorp, bioinformatics and the Sapio LIMS (laboratory information management system) play a critical role in the process of specimen accessioning, testing, and results.
LabCorp uses the Sapio LIMS in both dry and wet testing workflows to support its clinical lab. The dry testing workflow usually includes some or all the following:
- Intake review – confirm correct test was ordered and enter patient/specimen information
- Prior authorization – confirm insurance will pay for the test
- Informed consent – when required by state
- The wet testing workflow — of blood, saliva, or another specimen type — usually includes the following:
- Extraction – extract DNA from specimen and generate labels
- Assay prep – build plates, robot instructions, instrument worklists, and labels
- Analysis – file orchestration and automation, bioinformatics algorithm invocation, calculations quantification, and visualizations
- Interpretation – variant scoring, batch assessment, QC metric evaluation, technical review, director approvals
- Confirmations – confirm plate builds, robot instructions, instrument worklists, labels, and algorithm invocation
- Result processing – all specimen test data collected and held for lab director review of primary and confirmation data
The Role of Bioinformatics in the Clinical Lab
Angela Kenyon is the Director of Bioinformatics at LabCorp. She says her team, “wears many hats,” at LabCorp collecting, managing, and reporting on a wide range of diagnostic information and activities, including:
- Variant detection – structural variation (SV), copy number variation (CNV), other technically complex regions, failed segments, pathogenic or likely pathogenic (PLP) filtering, and confirmation bypass
- Algorithms – qPCR, dPCR, fragment analysis, Sanger, MLPA, dMLPA, exome, genome, and CNV
- Bioinformatics – annotations, plotting, data mining, dashboards, and automation
LabCorp’s bioinformatics activities dovetail with the cloud-based Sapio LIMS’s dry and wet testing workflows to streamline and automate information input and output across its multiple geographic diagnostic laboratory sites. Sample queues are standardized across all sites, so regardless of the location, each scientist views the same information in the same, color-coded format. According to Angela, “It’s all about making an efficient product and a really nice user experience that leads to better results for our patients.”
Continually Identifying and Removing Bottlenecks
Continuous process improvement at LabCorp involves ongoing identification and rectification of bottlenecks to further automate and streamline laboratory operations. Beyond initial system definition and installation, LabCorp has capitalized on two opportunities with the LIMS to enhance performance and improve efficiency:
- Automatic assignment of cases overnight – Previously, genetic counselors (GC) might review hundreds of cases at the start of each shift and manually assign some to themselves. This process, repeated shift after shift, was time consuming and inefficient. LabCorp worked with Sapio to integrate work schedules into the LIMS, which now automatically assigns cases to GCs each shift.
- Intake review batch complete process –Previously, GCs would select numerous specimens individually during intake review and then select complete workflow. Repeating this process again and again was tiresome and inefficient. LabCorp worked with Sapio to enable a batch selection of multiple specimens in a single operation.
“You’re not going to get everything right the first try,” says Angela, “but you want to keep sitting down with your user community and make sure that you’re evolving the product together.”
Alternative Work Locations with a Cloud-Based LIMS
During the COVID pandemic, the Centers for Medicare and Medicaid Services (CMS) modified regulations so people could work on patients from their home rather than a CLIA certified lab. Because the LIMS is cloud-based, Sapio was able to modify code so that when someone is logging-in a message pops up to pick his or her location. The LIMS controls user access based on the location selected and automatically provides the appropriate information, such as the address on reports.
Beyond the pandemic emergency, a remote option has helped LabCorp with workforce retention and enabled hiring from a new, expanded pool of candidates to whom a remote work option is attractive. “Having a LIMS support the fact that our users may or may not be working from the same place all the time and helping us track whether you’re in a CLIA site or not was a double win for our user community,” says Angela. CMS has extended the remote work option through at least the end of 2025.
Tracking Lab Efficiency with the LIMS
LabCorp uses the LIMS to track a range of laboratory performance metrics quickly and easily, eliminating the need to run multiple queries — further improving efficiency. According to Angela, “Sapio has made it really nice where we can prebuild management reports directly in the LIMS and generate a report with a single click.”
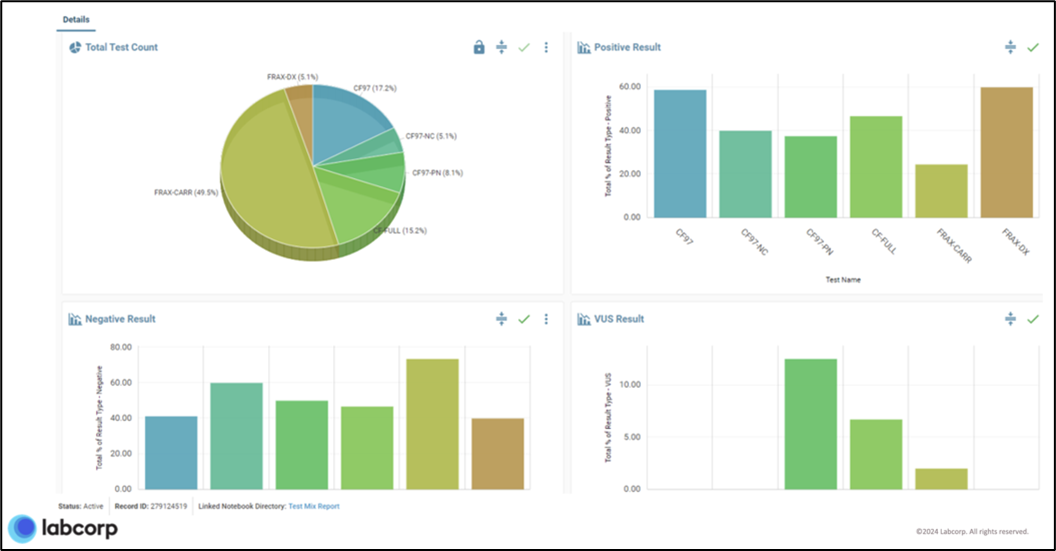
Reports are generated directly in a format appropriate for the audience, thereby avoiding time-consuming intermediate steps. For example, a report for R&D might use a boxplot or correlation plot to visually display Fold 80 coverage uniformity. While a visual display with pie and bar charts (example below) is used to answer a range of questions for lab management, including:
- How many tests did we run in a specific time (day, week, month)?
- What was the breakdown per test code?
- What was the assay failure rate?
- How many samples received positive, negative, or variant of unknown significance (VUS) results?
Conclusion: LabCorp’s Recipe for Success
When Angela is asked about LabCorp’s recipe for success, she says it’s a long list, but after eighteen months of experience, “If you’re hitting each of these points, you’re on the road to success.”
- Start by creating a strategic partnership between your LIMS team, bioinformatics group, and business users.
- Foster cross-functional collaborations to ensure you’re building reuseable components.
- Hold frequent live demonstrations for the user community and leadership.
- Capture new thoughts on enhancements in an organized fashion.
- Poll users for feedback regularly and test, test, test.
- Acknowledge that there will always be a new technology, variant type, and exception to consider and incorporate.
- Leverage trending tools to monitor performance of your assays and your LIMS.