Bioanalysis LIMS & ELN
A unified platform for flexible and reproducible bioanalytical studies.

Sapio’s Bioanalysis
LIMS & ELN adapts
to the way you work.
Sapio’s bioanalytical LIMS & ELN seamlessly manages your data, creating efficiencies and ensuring compliance for both small/large-molecule and multi-modal workflows in clinical and non-clinical studies.
Sapio bioanalysis offers complete traceability and control from study initiation to closeout, with built-in data validation, study design, and information management capabilities. Designed to maximize scientific autonomy while minimizing errors, our platform supports complex bioanalytical workflows and adheres to industry regulations.
The industry has seen steep growth in bioanalytical studies in recent years, but current market growth numbers indicate the boom has just begun. Projected to rise at a CAGR of 13.7% by 2030, bioanalysis plays an essential role in characterizing large and small molecules and evaluating their performance through pharmacokinetics and pharmacodynamics studies.
What is Laboratory Bioanalysis?
Bioanalysis in the lab involves quantitatively measuring and analyzing drugs, metabolites, and biomolecules in biological systems. It encompasses sophisticated techniques and instruments for detecting and quantifying substances in various biological samples, such as blood, urine, and tissues.
The process includes sample preparation, method development, validation, and data interpretation to support pharmacokinetic, pharmacodynamic, and toxicological studies. Bioanalytical work is crucial for understanding compounds’ absorption, distribution, metabolism, and excretion (ADME), ensuring the safety and efficacy of pharmaceuticals in clinical and preclinical research.
Sapio LIMS & ELN delivers a complete solution for the bioanalysis pipeline.
Master Assay Creation and Quality Control
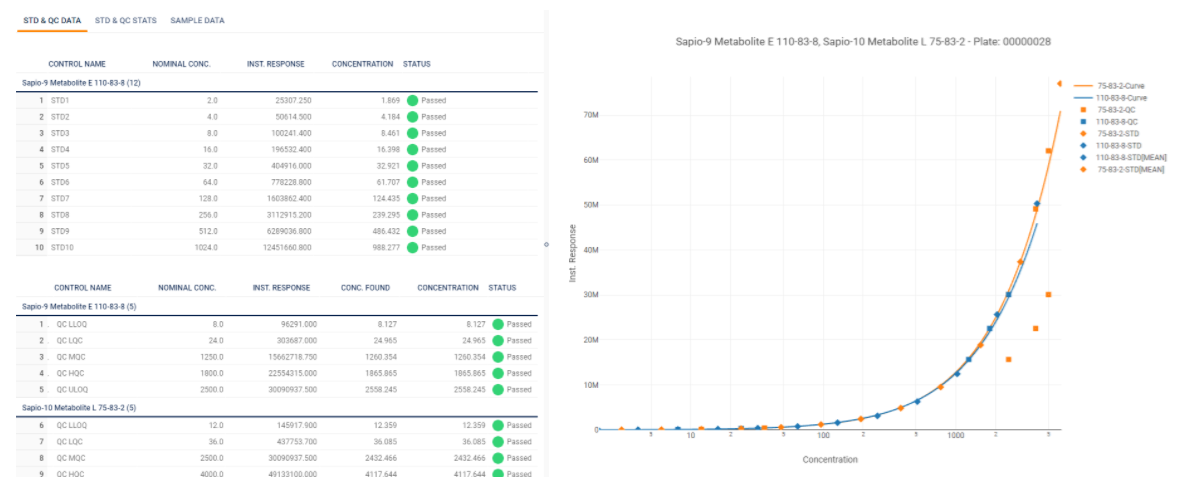
In a few simple steps, create a master assay in Sapio that can be used to enforce quality across all of your analytes. Ensure standards and QC samples meet the mark without exception using built-in curve fitting and statistics. Connect it all by scaling validation and QC from your experiments to your studies.
Configurable Workflow
Bioanalytical workflows often require adjustments to accommodate different experiments, sample types, and research objectives. Configurable workflows allow scientists to tailor protocols and methods without starting from scratch each time and then quickly modify and test new hypotheses. Stay agile with Sapio!
Traceability End to End
With Sapio, streamline every stage of your bioanalytical workflow with a robust and intuitive tracking system. From the moment samples are registered, you can manage their approval, receipt, and storage, ensuring traceability and compliance at every step. Gain unparalleled visibility with detailed sample lineage maps, showcasing the complete journey from collection to analysis.
Seamless Instrument Integration
Sapio offers seamless integration through APIs and real-time webhooks, enabling real-time communication with your lab systems. Receive instant notifications and trigger actions based on predefined events, ensuring seamless workflow orchestration. Sapio experts, both in-house and through our partner ecosystem, will assess your needs, configure your ideal solution, and deploy it professionally.
One experience, made for you
Sapio flexibly supports the entire bioanalysis pipeline without custom code—from study set up, to sample preparation and processing, method design and validation, execution and analysis. Use pre-built bioanalytical templates as a starting point and configure to your liking—no programmers needed.
Compliance Built-In
Ensure data integrity and regulatory compliance in your lab with our comprehensive GxP-compliant solution. Meeting FDA GLP, GCP, and GMP standards in the US, and OECD, EMA in Europe, our system guarantees high-quality data for regulatory submissions. It also ensures compliance with 21 CFR Part 11, FDA Bioanalytical Method Validation, and EMA guidance.
Manage studies seamlessly
Track dosing, operations, measurements, and samples over time with Sapio’s science-aware™ study management templates, analytics, and dashboards. Watch your study progress before your eyes using at-a-glance graphs, intuitive Gantt charts, and an integrated calendar view.
Experience why Sapio is the ideal software choice for bioanalysis.
Bioanalytical Features
Sapio comes with everything scientists need to support standardized, scalable bioanalysis studies. Here are some of the features that make Sapio the platform of choice for bioanalytical companies.
- Pre-built bioanalytical templates
- Instrument management
- Materials management
- Method development
- Reagent preparation
- Sample preparation
- STD & QC for calibration
- Method validation & execution
- Interactive 3D plater
- Reusable assays
- Science-aware™ data analysis and visualization
- GLP compliance and SOPs
- Curve Fitting
- Immunogenicity
Scientific leaders choose Sapio LIMS & ELN.








Do you have questions about Bioanalysis software?
We’d love to answer them.
Sapio Bioanalysis streamlines the entire bioanalytical process from assays to studies and statistics by offering a flexible, no-code solution with ready-made workflows and experiment templates. The platform supports detailed tracking of dosing, sample, and measurement scheduling, making the process simple and efficient.
Sapio Bioanalysis provides comprehensive study design capabilities, including sampling and measurement scheduling. It also features robust sample management with the ability to register samples, capture metadata, assign storage locations, and track the sample lifecycle. Additionally, the platform offers integrated tools for visualizing study data, such as graphs and Gantt charts.
Sapio Bioanalysis adheres to GLP guidelines and supports compliance with regulatory standards by tracking the master assay, subjects, treatment groups, and chain of custody throughout the lifecycle of a study. It also manages instrument calibrations, reagent preparations, and performs advanced analytics to ensure data integrity and accuracy.
Sapio Bioanalysis enhances method validation and data analysis through integrated analytics, such as linear and non-linear curve fitting. The platform aggregates data to form analytical statistics, including R squared, ISR Pass Rate, Mean, SD, % CV, and % Bias. These features ensure high standards of accuracy and reproducibility, essential for regulatory submissions and scientific research.
Bioanalysis Resources
Webinar
Mastering Bioanalytical Workflows
Brochure
Bioanalysis Application Sheet
Guide
A Science-Aware™ Approach to Lab Informatics
Allow us to show you how Sapio will supercharge your bioanalytical work.
Fill out the form to request your demo.